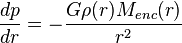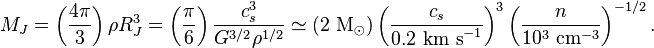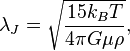What I’ve Learned:
“Jeans instability: The fancy-pants stuff behind every star.”
Science is hard. Most of it is obviously complicated and full of tongue-twisty words that exist only so some eggbrain jerkass can school you at Words with Friends. But it’s sneaky, too. Whenever some small bit of science seems simple and straightforward, there’s always something way harder and full of Greek-letter math lurking underneath. That’s how science gets you.
Take Jeans instability, for example.
Everything physicists tell you up front about Jeans instability makes perfect sense. You’ve got this lumpy stuff called Jeans mass, and a size called a Jeans radius. If the Jeans mass exerts too much pressure for a given Jeans radius, the system flies apart and the mass spreads all over.
We’ve all been there. Like an hour after after Thanksgiving dinner.
On the other hand, if the Jeans mass has too little pressure, then Jeans instability occurs and the system collapses in on itself.
Presumably in a little pile around your ankles. I can’t say I’ve personally had experience with this phenomenon. It sounds like one of those tragic Euro supermodel problems. Oh, those poor twiggy bitches.
All of this is well and good, until the physicists then tell you that none of this has anything to do with distressed Calvin Kleins, Levis 501s or high-waist super skinny Jordache denim jeggings — and is that last one actually a thing? Merciful Darwin help us all.
To physicists — who mostly wear plain, practical polyester pants, it turns out — Jeans instability is a whole other thing entirely. It’s a phenomenon named after British physicist Sir James Jeans — personal legwear preferences unspecified — and describes the conditions under which interstellar gas clouds collapse to form stars.
On the bright side, most of the above reasoning still holds true. If the outward pressure of the gas in a cloud of a given size is too great — because the gas is especially hot, for instance — then the pressure will overcome gravitational force, and gas will spill out everywhere.
Like I said, usually an hour after Thanksgiving dinner. That happened to me twelve years ago, and Grandma still won’t invite me back for holidays.
But if the gas is sufficiently cool, or the mass of the cloud unusually high for the space it’s in, then gravity wins out and the gas will collapse in on itself, eventually forming a discrete object called a protostar, and later a star. It’s the Jeans instability that predicts under what conditions this collapse will begin to occur.
(Presumably, it includes declining seconds on pumpkin pie. Again, I wouldn’t know. That would require a stronger cloud of gas than I.)
That’s the good news, in terms of simplicity. The bad news is, the original equation for Jeans instability has been found by later researchers to not be completely accurate for real-world predictions. Which might explain why people try to fit into pants two sizes too small. Also, that equation for Jeans instability looks like this:
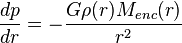
And to get the Jeans mass, you apparently solve this gibberish:
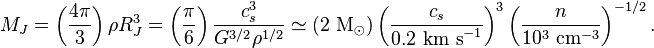
And the Jeans radius — more often called Jeans length — comes out the back end of this beast here:
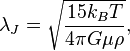
I don’t know what any of that means. I have trouble enough figuring out the right inseam to put in the form on the Wrangler website. What if the gas cloud is wearing a belt? Is there more instability if you acid wash first? And how do I convert the units for the gravitational constant into boot-cut?
I’m telling you. Science is hard.