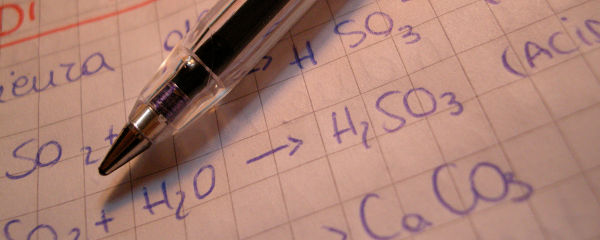What I’ve Learned:
“Transactinides: They’re not heavy. They’re your metals.”
The transactinides are a group of fifteen elements — fundamental building blocks of matter, like carbon or hydrogen or double-sided duct tape. But transactinides are pretty special elements, in a number of ways.
First, the transactinide elements are all radioactive, which means they spontaneously split apart into other elements, releasing energy in the process. What’s more, these elements are highly volatile — even the most stable break down within about twenty-eight hours. Much like that drama major you dated back in college.
Transactinides are also the heaviest elements known to exist — and none have ever been detected in nature. We’ve only found them in the laboratory, by cramming atoms of smaller elements together until they stick for a few seconds, like some kind of chemically-unstable PB&J.
(I don’t know what a PB&J decays into, exactly. Strawberry Pop-Tarts? A jelly doughnut? Uncrustables?
This is why you don’t see many snack-related analogies in chemistry textbooks. Clearly, sandwich science is still in its infancy.)
These elements are so bleeding-edge, they don’t even get real names until they’ve been produced in a lab and the results tested and repeated. At that point, a newly “confirmed” transactinide is usually named in honor of someone important to science. Like Rutherfordium was named after physicist Ernest Rutherford, or Seaborgium for a race of Doctor Who villains, I think, or Livermorium, which was named after something a bird said in an Edgar Allen Poe poem. Science is all over the map sometimes.
But before those fancy names, the more theoretical transactinides get systematic titles to identify them. These provisional names are built from Greek and Latin roots for numbers, smooshed together like the ephemeral atomic phenomena they describe. So the element with atomic number 113, for instance, is currently called ununtrium, while the heaviest transactinide, with atomic number 118, is ununoctium.
(Nobody in science really uses these names, for two reasons. First, it’s simpler to just say “element 118”. And second, nobody wants to spend their career trying to produce something that sounds like a disease you get from licking raw chicken meat.)
While most of the periodic table is well established at this point, physical chemists still work on transactinide elements — usually trying to produce the ones not yet confirmed. Just this week, element 117 — or ununseptium, if you prefer your science Gregorian chant-style — was confirmed by a lab in Germany. It was first synthesized by a joint American-Russian team in 2010, who fired a beam of heavy calcium isotopes into a bunch of berkelium atoms to get the job done.
That was a challenge in itself. Berkelium currently only exists on this planet as the result of synthesis experiments and “nuclear incidents” — like an H-bomb test, or Chernobyl disaster.
Also, berkelium’s half life is less than a year, so if the scientists couldn’t agree quickly about how to do the experiment, the berkelium they made for it would have already turned into something else.
So basically, this marks the only time in recorded history when Americans and Russians have gotten their shit together in short order to produce something good. From sammiches to glasnost, is there anything transactinides can’t do?